Spinal Cord Stimulators
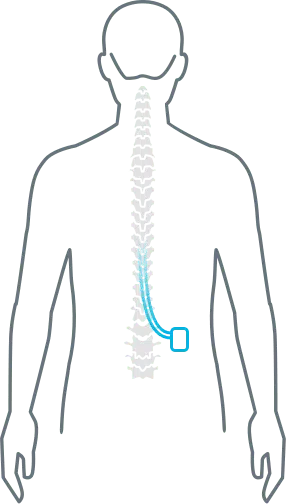
Spinal Cord Stimulators (SCS) are used in the treatment of chronic neck, arm, back and leg pain by disrupting pain signals before they reach the brain. The electrical pulses or stimulation is delivered by small electrodes on leads that are placed near the spinal cord and connected to a compact, battery-powered generator implanted under the skin. The procedure is outpatient and minimally invasive, where you will likely go home the same day.
Spinal Cord Stimulators have been around for over 35 years; however, the newest products offer major advancements in patient outcomes, including FDA-approval for use while driving, no tingling sensation that distracts you from pain, and reduced opioid use. If you and your doctor agree at your initial consultation that Spinal Cord Stimulation could work for you based on your condition, you will trial the device for a week before fully committing to an implant. A trial is a requirement before implant along with a behavioral health evaluation and physical.
A Patient’s Guide to the Spinal Cord Stimulator Process
What is a spinal cord stimulator?
A spinal cord stimulator is an implantable pain relief device that delivers electrical current to the spinal cord, which interrupts the pain signal that is traveling to the brain. A spinal cord stimulator is implanted for neck, back, arm, and leg pain. iSpine Clinics works with a wide range of medical device spinal cord stimulator manufacturers that offer a full range of therapies including a low-frequency current to replace the pain sensation with a mild tingling feeling called paresthesia. Other SCS devices use high-frequency or burst pulses to mask the pain with no tingling feeling.
Stimulation does not eliminate the source of pain. It simply changes the way the brain perceives it. As a result, the amount of pain relief varies for each person. Conversely, stimulation does not work for everyone. Some people may find the sensation unpleasant. Other people may not get relief over the entire pain area. For these reasons a trial stimulation allows you to try it for a week. If it doesn’t work for you, the trial wires can be removed, leaving no damage to the spinal cord or nerves.
How does a spinal cord stimulator work?
The doctors and pain specialists at iSpine will determine which device is appropriate based on the patient’s pain and medical history.
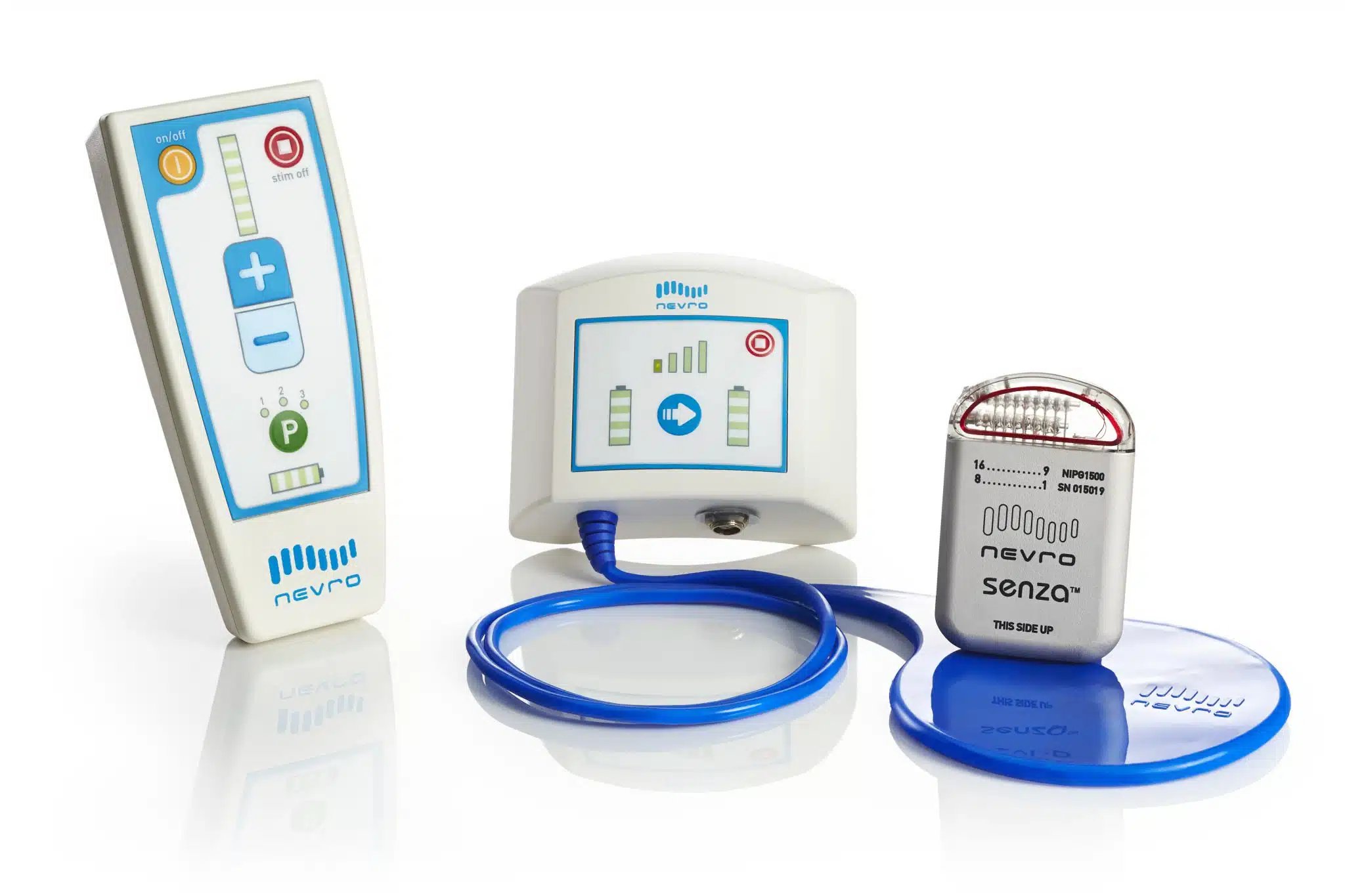
There are several types of SCS device systems. However, all have three main parts:
- A pulse generator with a battery that creates the electrical pulses, which is about the size of a man’s watch
- A lead wire with a number of electrodes (8-32) that delivers electrical pulses to the spinal cord.
- A hand-held remote control that turns the device on and off and adjusts the settings.
An SCS can help lessen chronic pain caused by:
- Chronic leg (sciatica) or arm pain: ongoing, persistent pain caused by arthritis, spinal stenosis, or by nerve damage.
- Painful Diabetic Neuropathy (PDN): Diabetic condition that can cause burning or stabbing pain, often in the legs or feet.
- Failed back surgery syndrome: failure of one or more surgeries to relieve persistent arm or leg pain, but not a technical failure of the original procedure.
- Complex regional pain syndrome (CRPS): a progressive disease in which patients feel constant, chronic burning pain, typically in the foot or hand.
- Arachnoiditis: painful inflammation and scarring of the protective lining of the spinal nerves.
- Other: stump pain, angina, peripheral vascular disease, multiple sclerosis, or spinal cord injury.
Requirements may include:
- Failed conservative treatments such as nonsteroidal anti-inflammatory medications, muscles relaxers or pain medication.
- Imaging: An MRI or CT scan within the last 12 months.
- Physical therapy: within the last 6-12 months, varies by insurance company.
- Psychological evaluation: within the last 6-12 months. The psychological evaluation is used to reveal any existing behavioral health problems.
All of these records must be provided to iSpine. We can assist you in obtaining these records by providing a release of information.
iSpine Clinics provides Consultations to determine if a Spinal Cord Stimulator is right for you
Explore the Twin Cities Metro Clinics where we evaluate patients for Spinal Cord Stimulators
*iSpine Clinics where Spinal Cord Stimulator procedures are conducted
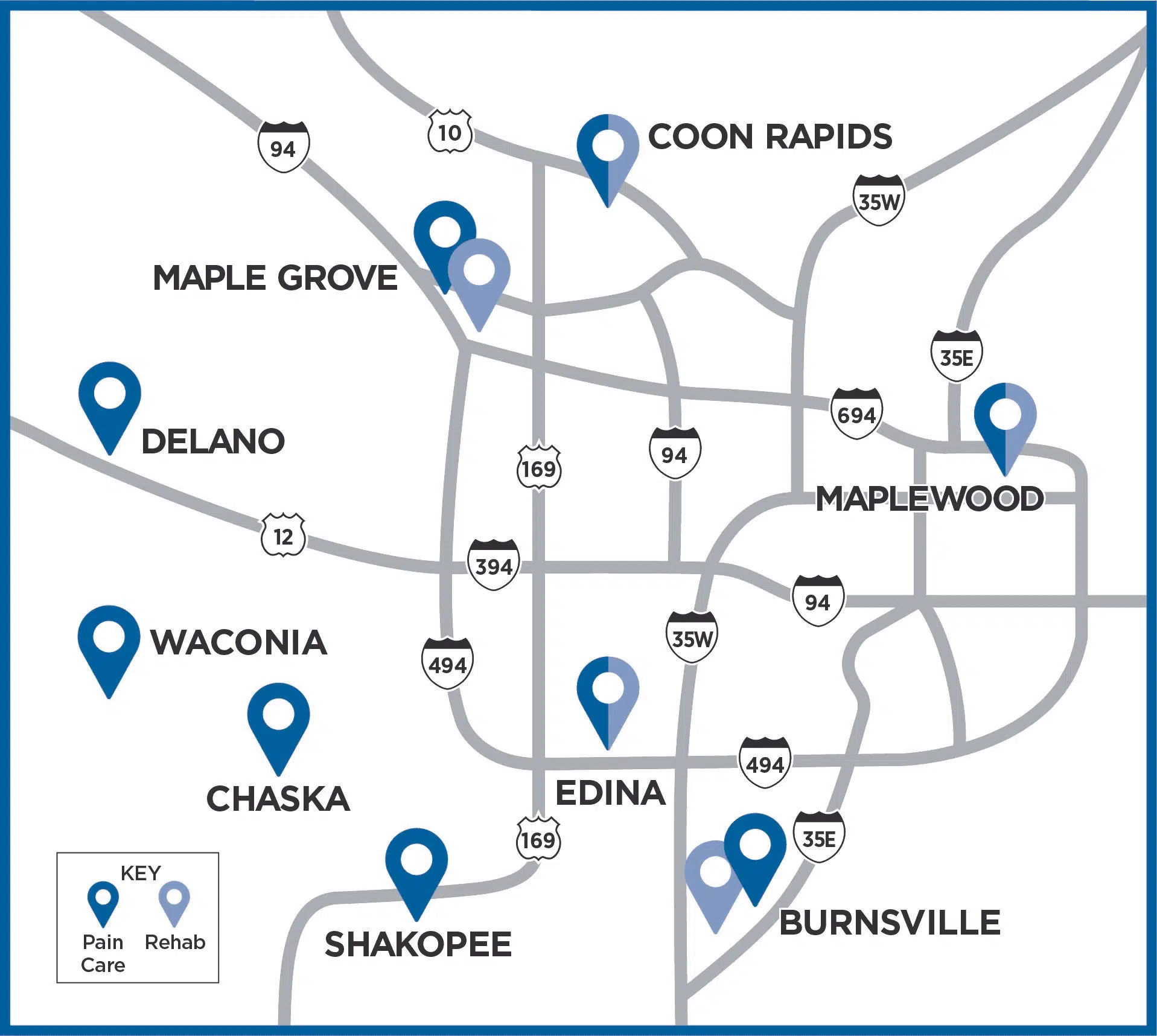
Dark blue pins represent iSpine Pain Clinic locations
Spinal Cord Stimulator: What to Expect
SCS trial process
Prior to the SCS trial, the patient will meet with the representative from the medical device manufacturer. The patient will be provided with information and questions will be answered.
SCS trial
The SCS trial is the placement of temporary leads. The patient will need a driver for this appointment. A representative from the manufacturer will be available during the SCS trial.
Lead pull
The lead pull typically happens after 3-7 days. At this appointment the patient and the provider will determine if the SCS trial was successful.
SCS implant process
Pre-op physical
The patient will contact their Primary Care Physician to schedule a pre-op physical within 30 days prior to the procedure. The results must be faxed to iSpine at 763-201-8192.
SCS implant surgery
The permanent lead and battery will be placed by the physician and is typically performed on an outpatient basis.
The patient will need a licensed driver to take them home and stay with the patient for the first 24 hours.
Post-op appointment
The patient will meet the device representative at iSpine Clinics to have the device turned on and programmed.
After spinal cord stimulator surgery
After the surgery has been completed, the patient will have some restrictions for 4-6 weeks, but can return to normal activities after that. The physician will discuss this in detail prior to the surgery.
The patient returns to the clinic one week after the surgery for a post-op appointment where they meet with a nurse and the manufacturer’s representative, who turns the device on and programs it. The representative will show the patient how to use the device and answer questions.
Spinal Cord Stimulator (SCS) Technologies
At iSpine Clinics, we work with a variety of medical device companies and tailor our product recommendations based on your unique case.
Saluda Medical’s Evoke® System
The Evoke® System is the first and only Smart spinal cord stimulation system (SCS) that allows therapy to be precisely tailored to meet your unique needs. It is the only FDA-approved system that constantly listens to your body, and automatically adjusts 4+ million times a day to maintain pain relief. The Evoke® System was approved by the FDA based on the most rigorous clinical study in the history of spinal cord stimulation. In the EVOKE clinical study, two-thirds of patients voluntarily reduced or eliminated their opioid medications. Nearly 8 in 10 patients experienced improvements in their ability to perform daily activities, and 6 in 10 patients showed clinically significant improvements in sleep (amount and quality).
Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomized, controlled trial. Lancet Neurol. 2020;19(2):123-134

Nevro HFX
Nevro’s HFX™ spinal cord stimulation (SCS) therapy offers superior results over traditional SCS for chronic back and leg pain. The HFX is clinically proven to offer substantial pain relief without the tingling, burning, pricking, or buzzing often felt in traditional SCS. In addition, the HFX is the only SCS system approved by the FDA to be used without patient restrictions of driving while receiving therapy. The HFX delivers electrical signals to the spinal cord to alter pain signals to the brain. The electrical pulses are delivered by small electrodes on leads that are placed near the spinal cord and are connected to a compact, battery-powered generator implanted under the skin.
Kapural L., et al. Novel 10-kHz High-frequency Therapy Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain. Anesthesiology, 123(4)
WaveWriter Alpha SCS™
https://www.pain.com/en/chronic-pain-solutions/spinal-cord-stimulation.html
The WaveWriter Alpha SCS system is unique in its ability to deliver multiple therapies simultaneously.¹ Some SCS therapies replace pain with a tingling sensation, others use a type you don’t feel at all. WaveWriter allows you to choose the therapy that is the best for you, or use both at the same time. It is designed to interrupt pain signals through your spinal cord to your brain. The WaveWriter Alpha SCS System features Boston Scientific’s unique, non-tingling FAST™ therapy. While other SCS systems use therapies that can take days to “wash in”, FAST is designed to provide immediate relief. In a recent clinical study, patients using FAST went from an average pain score of 6.5 to an average of 1.3 in a matter of minutes.² And, because FAST uses less energy than other forms of non-tingling therapy, it may help to reduce the amount of charging you have to do or to extend the life of a non-rechargeable system.³

References: 1. North, James, MD. WHISPER: A Multicenter, Prospective Randomized Controlled Trial Evaluating Subperception SCS at ≤ 1.2 kHz. Presentation at North American Neuromodulation Society (NANS), Las Vegas, NV, January 11-14, 2018. (N=70) 2. Clark S. Metzger, M. Blake Hammond, Jose F. Paz-Solis, William J. Newton, Simon J. Thomson, Yu Pei, Roshini Jain, Michael Moffitt, Luca Annecchino & Que Doan (2021) A novel fast-acting sub-perception spinal cord stimulation therapy enables rapid onset of analgesia in patients with chronic pain, Expert Review of Medical Devices, DOI: 10.1080/17434440.2021.1890580. (N=41) 3. FAST MOA computational modeling by Dr. Warren Grill’s lab at Duke University. Gilbert et al., Computational modeling predicts dorsal columns are involved in fast-acting sub-perception spinal cord stimulation (SCS). SFN 2021.
Abbott’s Proclaim™ XR
https://www.neuromodulation.abbott/us/en/chronic-pain.html
The Proclaim™ XR SCS system combined with the power of BurstDR™ stimulation therapy is Abbott’s latest advancement in neurostimulation therapy (also called Spinal Cord Stimulation). Not only does this system offer you superior¹ pain relief, but unlike other SCS systems that require frequent charging sessions to maintain therapy, the Proclaim XR SCS system is recharge-free. Meaning, you can get hassle-free pain relief with a battery that lasts up to 10 years at low dose settings² without ever needing to charge the system.
References: 1. When compared to traditional tonic stimulation. 2. Up to 10 years of battery longevity at the lowest dose settings: 0.6mA, 500 Ohms, duty cycle 30s on/360s off. NOTE: In neurostimulation therapy, “dose” refers to the delivery of a quantity of energy to tissue. Safety comparisons and specific dose-response curves for each dosage have not been clinically established. Refer to the IFU for additional information. Hassle-free means recharge-free.
Medtronic’s Intellis™ with DTM™ SCS
Medtronic’s DTM™ SCS therapy with Intellis™ includes the world’s smallest neurostimulator implant with a convenient wireless communication system that works with your life. The neurostimulator automatically adjusts the therapy as your body moves, and offers fast, convenient recharging going from empty to full in approximately one hour.


